Do you know when Paxlovid should be used to treat COVID-19? Are you aware of the reasons for the mixed results of its phase 2 and phase 3 clinical trial data versus its real-life studies? Do you know what the most significant concern about Paxlovid is for its future application in treating COVID-19?
Reputed as a so-called “game-changer” oral antiviral pill to treat COVID-19, Paxlovid can prevent hospitalization and death in people who are at high risk of severe COVID-19. However, you should know that the research findings on Paxlovid are not always what they seem to be.
We will provide a balanced, unbiased review related to Paxlovid’s development history, clinical trial and real-world effectiveness data, and the drug’s advantages and limitations. We will also clarify the connection between oral antivirals and human immunity.
Summary of Key Facts
- Paxlovid Is Not Yet Approved by the FDA
- Paxlovid Should Be Used Soon After Virus Infection
- Clinical Trial: 89 Percent Efficacy With Side Effects of Dysgeusia and Diarrhea
- Paxlovid Doesn’t Work in Younger Patients
- In a Real-World Study, Paxlovid Has Shown Limited Effectiveness
- Finding “Treatable” Patients Has Proven Challenging
- Drug Resistance Is a Major Concern
- Another Major Concern Is Paxlovid’s Interaction With Other Drugs
- Natural Immunity Influences the Success of Paxlovid and Other Antivirals
Pfizer’s Paxlovid contains two active ingredients. The first is nirmatrelvir (PF-07321332), a protease inhibitor that interrupts the viral replication cycle.
The action of viral protease is like a pair of scissors in the hands of a tailor. The protease can cut the long synthesized viral protein (like a piece of cloth) into various fragments with different functions. The virus will combine these protein fragments into a complete virus particle.
When the protease of the virus is inhibited, the virus is not able to replicate successfully; thus, protease is often treated as a therapeutic target by the pharmaceutical industry.
The other active ingredient of Paxlovid is an old HIV drug, ritonavir. Ritonavir is an HIV protease inhibitor that can help slow down the metabolism or breakdown of nirmatrelvir, thus maintaining nirmatrelvir’s effective concentrations.
1. Paxlovid Is Not Yet Approved by the FDA
On Dec. 22, 2021, the FDA issued an Emergency Use Authorization (EUA) for Paxlovid (nirmatrelvir tablets co-packaged with ritonavir tablets) to treat mild-to-moderate COVID-19.
On June 30, 2022, Pfizer filed a New Drug Application (NDA) with the FDA, seeking approval for Paxlovid. As of today, however, it has not been approved by the FDA for the treatment of COVID-19.
2. Paxlovid Should Be Used Soon After Virus Infection
A group of researchers, mainly from Pfizer Worldwide Research, published an article in Science on Nov. 2, 2021, about the discovery and characterization of Paxlovid. In vitro antiviral activity of Paxlovid has been evaluated in multiple cellular models. In vitro testing showed that Paxlovid demonstrated potent antiviral activity against SARS-CoV-2, MERS-CoV, and other similar coronaviruses.
However, the researchers noted that Paxlovid should be given very soon after a subject is infected with COVID-19.
When given to mice as early as four hours after infection with SARS-CoV-2, a 300 or 1,000 mg/kg treatment of Paxlovid was effective in reducing the SARS-CoV-2 viral load in the lungs.
This means Paxlovid should be taken as early as possible post-virus infection. That is also the rationale for the inclusion criteria: only patients within five days of symptom onset were recruited in phase 2 and phase 3 clinical trials. In other words, if the viral infection is in a late stage and the illness is more severe, Paxlovid may not be as helpful as it is for early infection.
It is worth mentioning that the start time of giving Paxlovid treatment, four hours after the virus infected animals, was even shorter than another antiviral, molnupiravir, which was dosed at 12 hours and 36 hours after virus infection in animals.
3. Clinical Trial: 89 Percent Efficacy With Side Effects of Dysgeusia and Diarrhea
The findings of phase 2–3 double-blind, randomized, controlled trial supported by Pfizer were published on Feb. 16, 2022, in the New England Journal of Medicine.
The trial involved 2,246 symptomatic, unvaccinated, non-hospitalized adult patients who were at high risk for developing severe COVID-19 symptoms, and symptom onset was no more than five days. They were randomly selected to receive either Paxlovid 300 mg with other standard care or a placebo with other traditional medicine twice a day for five days.
The final analysis, involving 1,379 patients, showed that Paxlovid reduced the risk of COVID-19-related hospitalization or death by 89 percent, compared to the placebo group when given less than five days after symptom onset.
The main side effects observed with Paxlovid vs. control were dysgeusia (a taste disorder, 5.6 percent versus 0.3 percent) and diarrhea (3.1 percent versus 1.6 percent), both higher than the placebo group. This indicates potential side effects on the neurological and gastroenterological systems.
Again, consistent with the development concept of this drug and aligned with its animal data, the drug has to be taken at an early stage of infection. Most patients (66.3 percent) received the first dose of the trial drug or placebo within three days after the onset of symptoms.
In the real world, not many patients can take the drug in the first onset days, especially during the current Omicron era, as most patients may view their symptoms as a common cold and may not be aware of having contracted COVID-19.
4. Paxlovid Doesn’t Work in Younger Patients
A large-scale observational, retrospective cohort study involving more than 100,000 subjects conducted in Israel during the Omicron-surge phase was published in the New England Journal of Medicine on Sept. 1, 2022.
This study was based on data obtained from a large health care organization covering approximately 52 percent of the Israeli population.
The research took place while the Omicron variant was dominant; the study period started on Jan. 9, 2022, and ended on March 31, 2022.
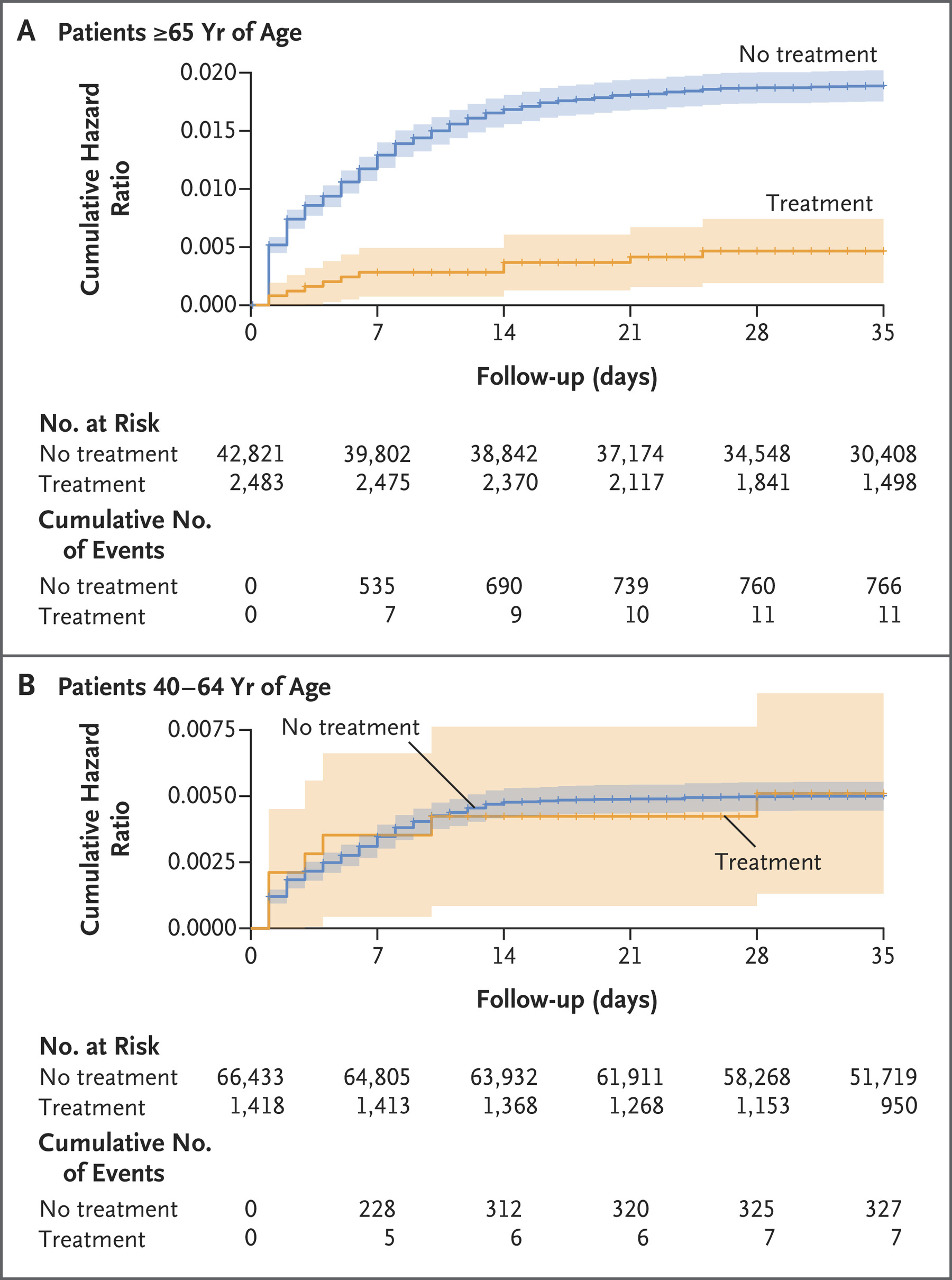
Researchers found that among patients 65 years of age or older, Paxlovid can lower the risk of hospitalization by 73 percent, and reduce the risk of death by 79 percent. There was, however, no evidence of a benefit found in 40- to 64-year-old adults.
5. Finding “Treatable” Patients Has Proven Challenging
We may wonder why Paxlovid works in elderly patients but not in the younger group of people in the Israel study.
Paxlovid has a narrow treatment window (five days after symptom onset) and a relatively short treatment period (five days only). Phase 2 and 3 trials are conducted in a selected group of patients in a controlled study setting. However, real-life studies may not be able to replicate the same criteria as phase 2 and phase 3 trials do—all this results in limitations for the clinical application of Paxlovid and mixed efficacy data.
First, the drug has to be taken by a patient as early as possible after SARS-CoV-2 infection. This is based on a list of factors involving the drug’s mechanism of action, development concept, preclinical study dosing timing, and the phase 3 study design. It’s also common sense that earlier treatment offers a better outcome.
However, it has been reported by a Johns Hopkins study that the “gold standard” COVID-19 PCR test has false negative rates ranging from 20 to 66 percent. The false negative rate of the SARS-CoV-2 PCR test is much higher during earlier infection days than later. It can be up to 100 percent on the first day and down to 20 percent on the eighth day of exposure (typically the third day of symptoms).
A systematic review of 34 studies enrolling 12,057 COVID-19-confirmed cases revealed that up to 54 percent of COVID-19 patients might have an initial false-negative RT-PCR result.
This results in a dilemma: on the one hand, we have to start treatment. On the other, the high false-negative rate hampers early Paxlovid application in the maximal patient population.
Paxlovid is not indicated for treating COVID-19 patients who require hospitalization due to severe or critical COVID-19 diseases. Based on previous clinical trial data, none of those trials were conducted in severe COVID-19 patients, so it is essential to bear this point in mind.
Furthermore, Paxlovid is not indicated for mild-to-moderate COVID-19 patients. Patients must present a high risk of progressing to severe diseases, including but not limited to older age, underlying medical conditions of cancer, diabetes, chronic lung diseases, or chronic kidney diseases.
Additional data support its use past five consecutive days. These factors together indicate that finding suitable test subjects has proven difficult.
6. In a Real-World Study, Paxlovid Has Shown Limited Effectiveness
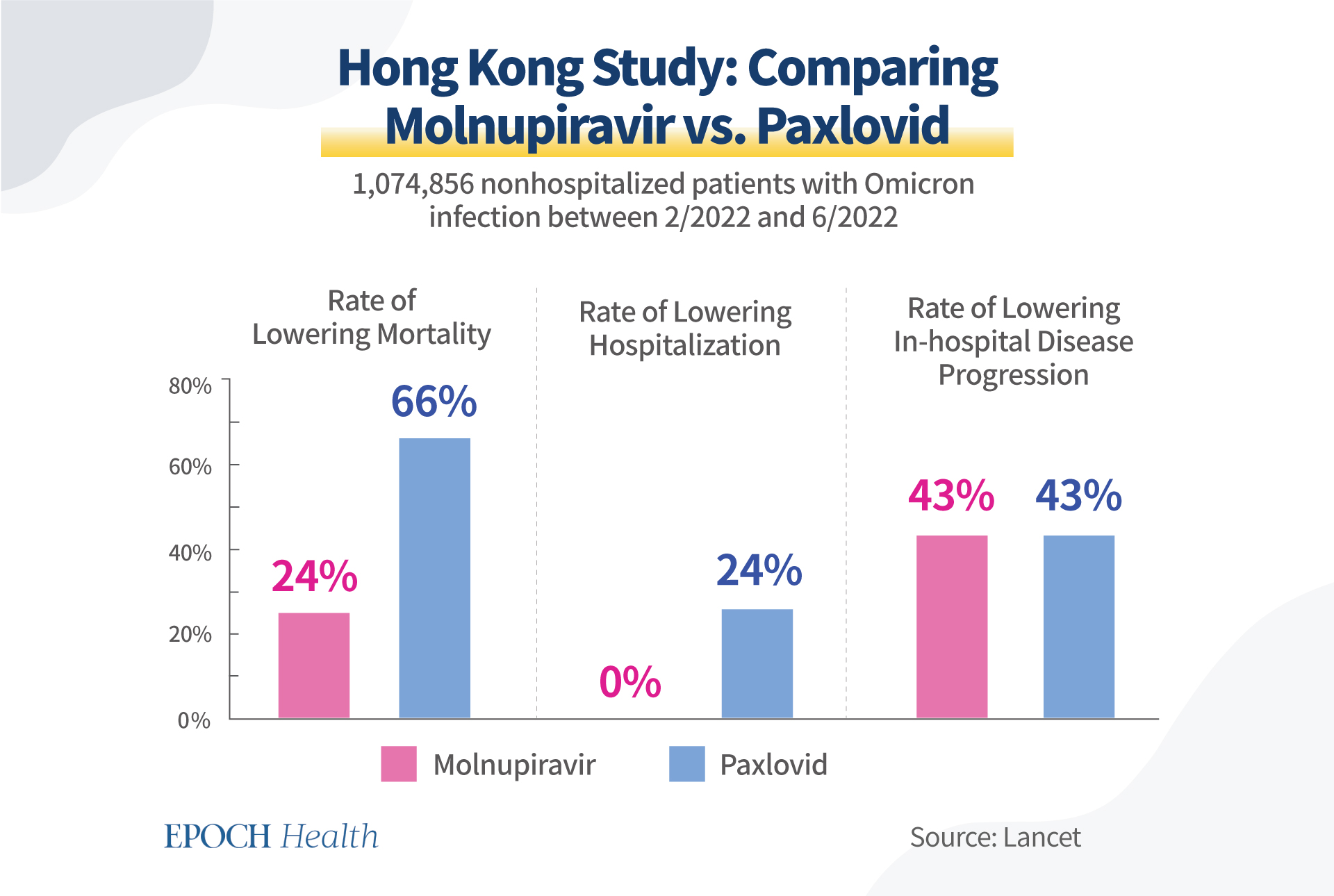
In October 2022, Hong Kong University researchers compared the clinical effectiveness of two oral antiviral drugs, Paxlovid and molnupiravir, among Hong Kong residents. The researchers’ observational study was published in The Lancet.
Between Feb. 26 and Jun. 26, 2022, among the 1,074,856 non-hospitalized patients infected with Omicron, 6,464 were treated with Paxlovid and 5,383 with molnupiravir. Compared to the Paxlovid group, the molnupiravir group consisted of older and more unvaccinated patients.
It is worth noting that these two antivirals were not compared directly in this study, but each was compared to its control group with matching patient conditions.
Paxlovid reduced the mortality rate by 66 percent versus the control, while molnupiravir lowered the mortality rate by 24 percent versus its own control.
Molnupiravir did not lower hospitalization rate, while Paxlovid lowered the hospitalization rate by 24 percent.
Molnupiravir lowered in-hospital disease progression by 43 percent, while Paxlovid also decreased it by 43 percent.
None of these rates look as good as Paxlovid’s phase 2 and phase 3 clinical trial data. One of the most likely reasons is how well the narrow treatment window of the drug has been followed, i.e., only five days after symptom onset.
7. Drug Resistance Is a Major Concern
Antiviral treatments are often associated with the development of drug-resistant viruses. There is a well-known saying in the antiviral community: “No antiviral, no resistance.”
A virus is a cunning microorganism. When you add pressure to its replication cycle, a virus will typically find a way to detour by mutating and will manage to survive. This is the primary mechanism of antiviral resistance.
There are already quite a few lab research studies indicating SARS-CoV-2 could mutate to bypass the drug target, i.e., reducing the efficacy of the drug in actual clinical usage, as reported by Science in June 2022.
In this sense, Paxlovid is no different than other antivirals.
Even though researchers reported that nirmatrelvir remained effective in multiple variants of SARS-CoV-2, including Alpha, Beta, Delta, Gamma, Lambda, and Omicron, as well as the original strain, this does not mean that the drug will be effective against future variants of the virus.
Two research groups have independently shown that SARS-coV-2 quickly gains the ability to avoid nirmatrelvir’s attack.
One study was led by virologist Dirk Jochmans from Belgium, who found that after a dozen rounds of nirmatrelvir treatment, SARS-CoV-2 developed three mutations—at positions 50, 166, and 167 of the critical protease Mpro—that reduced the virus’ susceptibility to nirmatrelvir 20-fold.
Judith Margarete Gottwein led the other study at the University of Copenhagen. She spotted resistance-conferring mutations at similar positions 50 and 166 in Mpro, conferring 80-fold reduced susceptibility to nirmatrelvir.
What’s more shocking is the fact that two of the mutations (166 and 167) flagged by the Belgian group were already reported to be circulating in people according to a preprint of research findings by United States scientists posted on May 30, 2022.
As a result of this concern, patients under the use of Paxlovid should be regularly monitored for antiviral resistance, especially when signs of rebound or reinfection appear.
8. Another Major Concern Is Paxlovid’s Interaction With Other Drugs
CYP3A breaks down Paxlovid. CYP3A is one of the most important enzymes in our liver and digestive tract, which play a significant role in breaking down the drug.
Potent CYP3A inducers will reduce the drug exposure of Paxlovid, resulting in loss of response to Paxlovid treatment and increased risks of drug resistance.
Paxlovid is contraindicated with a long list of drugs of CYP3A inducers, partially due to the concern of potential antiviral resistance.
CYP3A inducers include glucocorticoids, rifampin, carbamazepine, phenobarbital, and phenytoin. A list of contraindicated drugs is available here. Patients must be made aware of these potentially risky interactions before they take Paxlovid.
9. Natural Immunity Influences the Success of Paxlovid and Other Antivirals
Even though we applaud the efforts by the pharma industry to develop antivirals, we should not forget those limitations. Furthermore, a well-functioning immune system is necessary for an antiviral drug to exert its effect.
The main merit of antiviral drugs is that when the body’s immune system is not strong enough, external drugs can temporarily inhibit the replication of the virus, giving our natural defense system some time to recover to its full strength.
Meanwhile, we should not neglect the ability of our bodies to produce antiviral substances. Interferon, for example, is produced by many immune cells (white blood cells, NK, NKT, and T cells). As the name implies, through “interference,” it achieves antiviral effects. Interferon is like a commander, giving instructions to coordinate various cells and signaling pathways to work together to fight a virus.
There are many ways to strengthen the amount and maximize the potential of the natural antiviral substances that our bodies make.
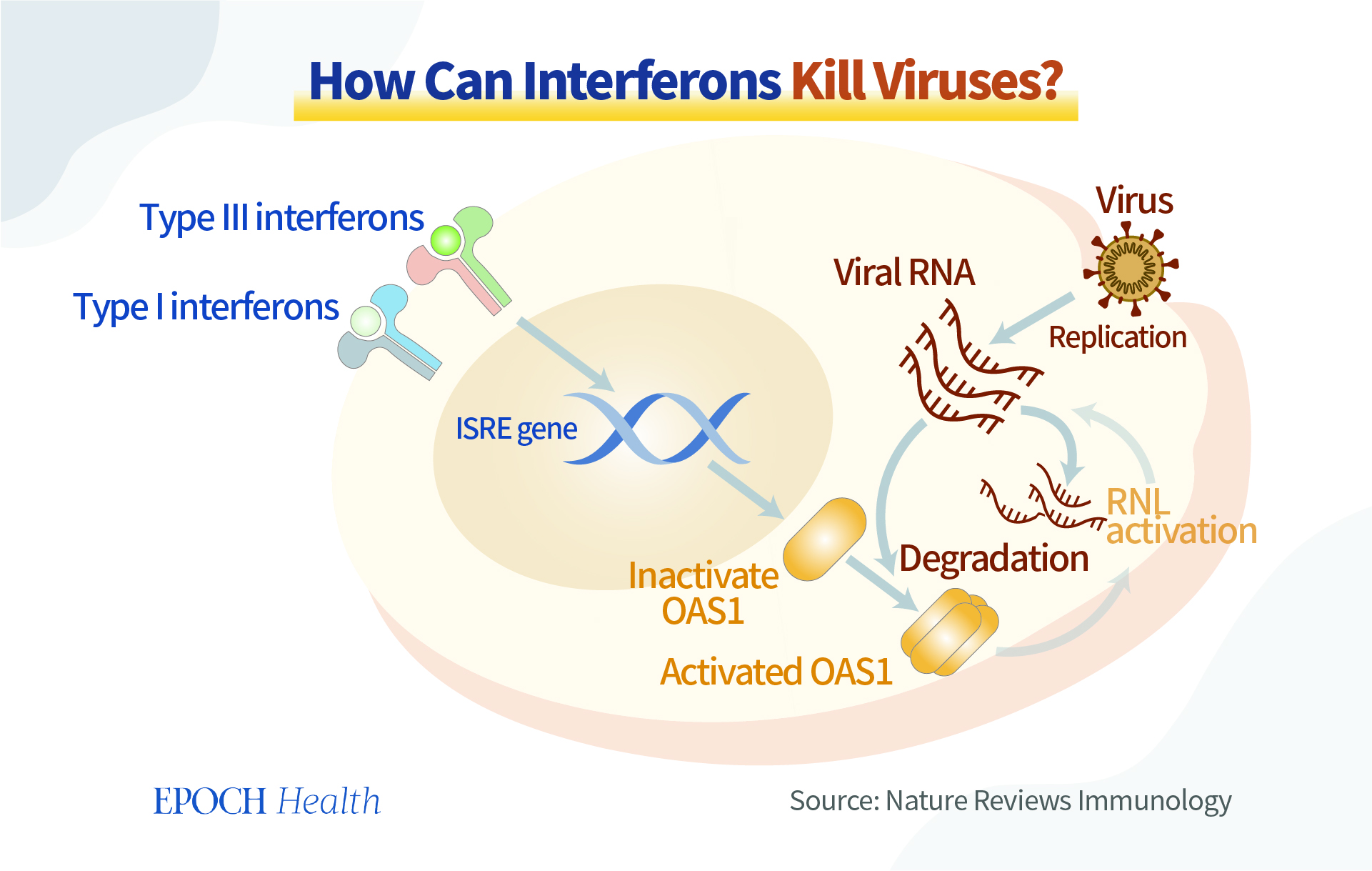
Natural immunity is an endogenous antiviral force in our body, like a joint force ready to fight against invading pathogens. If we are mindful enough to nourish our immunity in peaceful times, the defense mechanisms will work well when the “war” comes.
In summary, we should calmly view the strengths and limitations of antiviral drugs. On the one hand, we expect antiviral drugs to work well; on the other hand, we must enhance our immune system’s power to resist the virus in a more natural, systematic, dynamic, and resourceful manner.
Please share this.
No comments:
Post a Comment